Abstract
Follicular lymphoma (FL) is the most common indolent B cell lymphoma with a typically favorable outcome. However, ~20% of these patients have an early relapse with a poor prognosis. Thus far, efforts to identify factors that predict survival have been unsuccessful. However, we and others have shown the prognostic relevance of the tumor microenvironment (TME) and provided initial evidence for the role of specific genetic alterations in shaping different environments with highly dissimilar clinical courses. We have also identified three distinct molecular clusters, one of which was characterized by an IRF4 signature, an unfavorable TME and a poor outcome.
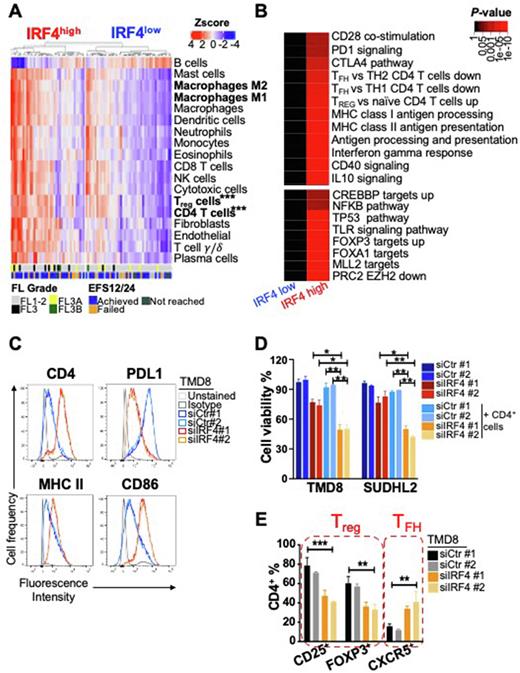
The master regulator IRF4 plays a critical role in terminal B cell differentiation. To investigate whether IRF4 modulates immune signatures in the TME, we integrated RNA-seq, mass cytometry (CyTOF) and whole exome sequencing (WES) of 88 newly diagnosed FL grade 1-3 patients. Using an established gene set that identifies the IRF4 signature(Shaffer et al, Nature 2008), we first performed singscore gene set scoring to discriminate IRF4high and IRF4low categories. IHC analysis of IRF4 expression in 20 samples with IRF4 high and low transcriptional signature validated this classification, with excellent concordance. Using CIBERSORT, we found that FLs with IRF4high expression had a strikingly different TME compared to IRF4low tumors. Specifically, there was a significant enrichment for CD4+ T cells (p<0.001), Treg cells (p<0.001), as well as M1 (p=0.027) and M2 macrophages (p<0.001, A). In line with the transcriptomic data, CyTOF analysis performed in 85 out of 88 FL cases showed that CD4+ (p=0.01), Treg (p=0.01) and TFH (p=0.03) cells differed significantly in IRF4high vs IRF4low FLs. Moreover, a CITRUS cluster analysis identified three significantly different clusters, including TFH exhausted and Treg cells.
RNA-seq of these 88 FL tumors revealed distinct transcriptomic profiles (FDR<0.05) according to IRF4 status with upregulation of 3,448 genes and downregulation of 2,907 genes. Pathway analysis using the Molecular Signature Database (MSigDB) revealed striking enrichment for (i) direct IRF4 targets (FOXP3, FOXA1); (ii) NF-kB targets (TP53, TLR signaling); and (iii) signatures associated with immune signaling including co-stimulatory (CD40 signaling) and inhibitory signaling (PD1 signaling), antigen presentation (MHC class I and II), upregulated Treg and downregulated TFH signatures (B). To investigate the somatic mutations and copy number alterations associated with differential IRF4 expression, we performed WES of purified B cells from the same 88 FLs and further expanded this cohort for a total 126 FLs. We found enrichment for mutations in B2M (which is a component of MHC class I) in IRF4high vs IRF4low FLs (p=0.024). The same cases showed a negative correlation for CREBBP loss-of-function mutations (which modulates MHC class II; p=0.008).
Silencing of IRF4 in TMD8 and SUDHL2 cells (ABC lymphoma cells with high IRF4 expression) by small interference RNA (siIRF4) increased CD40 and MHC class II expression while downregulating PDL1 at the transcriptional and protein levels (C). Conversely, ectopic expression of IRF4 in OCI-LY19 cells (GCB lymphoma cells with low IRF4 expression) generated the opposite effect. Using a luciferase-based promoter assay, we ruled out NF-kB-mediated control of IRF4 effects, as selective inhibition of IRF4 did not alter NF-kB luciferase activity. To investigate whether IRF4 silencing could trigger anti-lymphoma immunity, we co-cultured CD4+ T cells with TMD8 and SUDHL2 cells transfected with siIRF4 or siCtr for 5 days. As expected, IRF4 knockdown in these cells resulted in decreased cell viability, however this cytotoxic effect was significantly increased in the presence of CD4+ T cells, consistent with T cell-direct tumor killing (D). Notably, silencing of IRF4 in lymphoma cells doubled the number of TFH cells while halving the Treg cells compared to siCtr (E). In support of the T cell activation, ELISpot for IFNγ showed 8-10-fold increase when B cells were transfected with siIRF4. Collectively, we found that FLs with IRF4high expression are associated with a suppressive TME and selective IRF4 silencing restores anti-lymphoma T cell immunity. Further investigation is warranted to identify the mechanisms by which IRF4 controls tumor immunity to develop precision therapies for this unfavorable population.
Disclosures
Shlomchik:BlueSphere Bio: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: CSO, Founder; Anaptys Bio: Consultancy; SQZ Biotech: Other: SAB; Third Rock Ventures: Consultancy. Novak:Bristol Myers Squibb: Research Funding. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal